Questions and Answers
Questions and Answers
IARC Monographs Volume 127: Some Aromatic Amines and Related Compounds
From 25 May to 12 June 2020, the International Agency for Research on Cancer (IARC) convened a working group of 19 scientists from 9 countries to evaluate the carcinogenicity of some aromatic amines and related compounds. Because of the coronavirus disease (COVID-19) pandemic, the meeting for Volume 127 was held remotely. The outcomes of the assessments were published in a summary article in The Lancet Oncology on 25 June 2020. The full assessments will be published in Volume 127 of the IARC Monographs on the Identification of Carcinogenic Hazards to Humans.More information about the meeting is available on the IARC Monographs website:
https://monographs.iarc.fr/iarc-monographs-meetings/iarc-monographs-volume-127/.
1. What are the results of the evaluation?
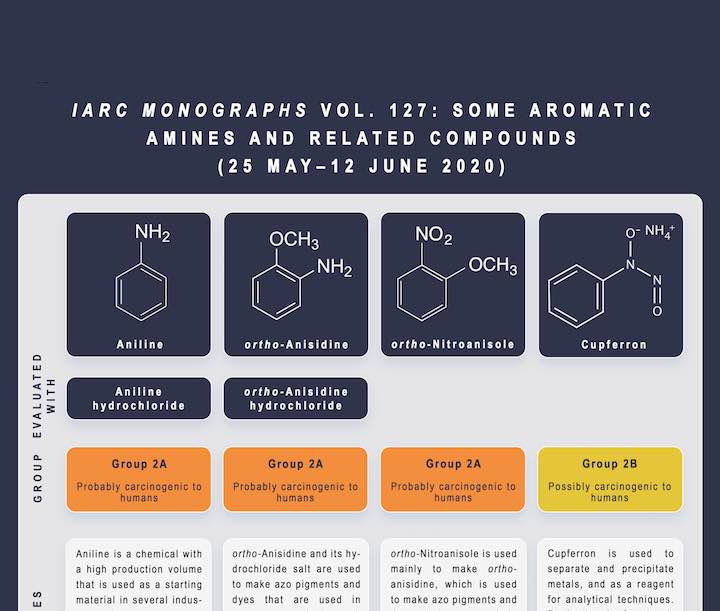
Aniline, ortho-anisidine, and ortho-nitroanisole were classified as probably carcinogenic to humans (Group 2A) on the basis of strong evidence that each agent belongs, on the basis of mechanistic considerations, to a class of aromatic amines for which several members of this class of chemicals (e.g. ortho-toluidine, 2-naphthylamine, and 4-aminobiphenyl) have been classified by the IARC Monographs as carcinogenic to humans (Group 1).
The classification is based on the similarities of these chemicals to other carcinogenic aromatic amines regarding how they are activated to DNA-binding electrophiles, their genotoxicity, and their target organs of carcinogenicity in chronic animal bioassays. There was strong evidence in experimental systems that these agents are genotoxic and exhibit other essential properties of human carcinogens (called key characteristics of carcinogens). There was also sufficient evidence of carcinogenicity in experimental animals. For each of these agents, the evidence for cancer in humans was inadequate.
Aniline hydrochloride and ortho-anisidine hydrochloride were also classified as probably carcinogenic to humans (Group 2A) because they are in equilibrium with the parent compounds in the body.
Aniline was previously evaluated as not classifiable as to its carcinogenicity to humans (Group 3), in IARC Monographs Volume 27, Supplement 7.
ortho-Anisidine was previously evaluated as possibly carcinogenic to humans (Group 2B), in IARC Monographs Volume 73 (1999).
ortho-Nitroanisole was previously evaluated as possibly carcinogenic to humans (Group 2B), in IARC Monographs Volume 65 (1996).
Cupferron was classified as possibly carcinogenic to humans (Group 2B). The classification was based on:
. sufficient evidence of carcinogenicity in experimental animals
. strong evidence in experimental systems that cupferron exhibits essential properties of human carcinogens (called key characteristics of carcinogens); cupferron is genotoxic.
Cupferron was not previously evaluated by the IARC Monographs programme.
2. Who is exposed to these agents and how?
Aniline is a chemical with a high production volume, and its main industrial use is as a starting material for one type of isocyanate, a chemical that is used to produce polyurethane foam. In lesser quantities, it is used to produce rubber-processing chemicals, dyes, colouring agents, inks, and certain pharmaceutical drugs. It has been detected in tattoo ink and in some pen inks; however, tobacco smoke is thought to be the main source of exposure in the general population. Aniline has been detected in drinking-water near contaminated sites. Exposure information was very sparse for all scenarios, particularly in low- and middle-income countries; however, the available data indicate that occupational exposure is higher than environmental or consumer-product exposure. Use of aniline seems to be moving towards low- and middle-income countries. Very little exposure information was found globally for aniline hydrochloride, which can be used as a flux in brazing or soldering operations.
ortho-Anisidine is a chemical that is used as an intermediate in the manufacture of dyes and pigments for consumer products such as textiles, printing paper, and cardboard, and in the production of pharmaceuticals. It is also identified in tobacco smoke and in wastewater and has been detected in drinking-water and during product testing of textiles and certain consumer products.
In addition to occupational exposure, individuals may be exposed to ortho-anisidine present in the environment. It is also present in tattoo ink and has been detected in the urine in the general population. Very little production or exposure information was available. ortho-Anisidine hydrochloride is not produced in large quantities. It is used as an intermediate chemical to make dyes and pigments and has other industrial uses. No exposure information was found for ortho-anisidine hydrochloride.
ortho-Nitroanisole is reduced to ortho-anisidine, an important dye intermediate. It is an industrial precursor for ortho-anisidine. There is potential occupational exposure, but very little published information was found. ortho-Nitroanisole was also detected in water samples and sediments worldwide.
Cupferron is used to separate and precipitate metals, and as a reagent for analytical techniques. Production of cupferron is found primarily in East Asia and India, and suppliers are based mostly in China and the USA. There is potential occupational exposure among workers engaged in analytical or research studies involving the manufacture or use of cupferron, but no published exposure information was available.
For all four agents, exposures to workers are expected to be higher than exposures in the general population.
3. What does the classification mean in terms of risk?
The classification indicates the strength of the evidence that a substance or agent causes cancer. The IARC Monographs programme seeks to identify cancer hazards, meaning the potential for the exposure to cause cancer. However, it does not indicate the level of cancer risk associated with exposure at different levels. The cancer risk associated with substances or agents that are assigned the same classification may be very different, depending on factors such as the type and extent of exposure and the size of the effect of the agent at a given exposure level.
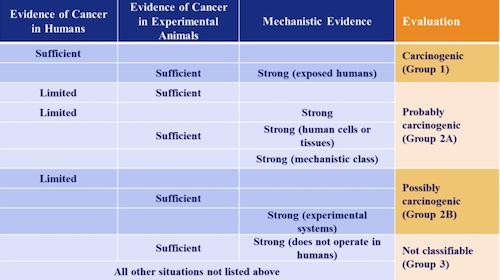
4. What are the different strength-of-evidence evaluation groups?
5. What are the different classifications of agents?
Group 1: The agent is carcinogenic to humans. This category is used when there is sufficient evidence of carcinogenicity in humans. In other words, there is convincing evidence that the agent causes cancer. The evaluation is usually based on epidemiological studies showing development of cancer in exposed humans. Agents can also be classified in Group 1 based on sufficient evidence of carcinogenicity in experimental animals supported by strong evidence in exposed humans that the agent has effects that are important for cancer development.
Group 2: This category includes agents with a range of evidence of carcinogenicity in humans and in experimental animals. At one extreme are agents with positive but not conclusive evidence regarding carcinogenicity in humans. At the other extreme are agents for which evidence in humans is not available but for which there is sufficient evidence of carcinogenicity in experimental animals. There are two subcategories, indicating different levels of evidence.
Group 2A: The agent is probably carcinogenic to humans. This category is used when there is limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals. Limited evidence of carcinogenicity means that a positive association has been observed between exposure to the agent and cancer but that other explanations for the observations (technically termed chance, bias, or confounding) could not be ruled out.
Group 2B: The agent is possibly carcinogenic to humans. This category is used when there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when the evidence regarding carcinogenicity in humans does not permit a conclusion to be drawn (referred to as inadequate evidence) but there is sufficient evidence of carcinogenicity in experimental animals.
Group 3: The agent is not classifiable as to its carcinogenicity to humans. This category is used most commonly when the evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Limited evidence of carcinogenicity in experimental animals means that the available information suggests a carcinogenic effect but is not conclusive.
6. How was the evidence reviewed?
During an IARC Monographs evaluation, experts critically review the scientific evidence according to strict criteria, which focus on determining the strength of the available evidence that the agent causes cancer. These criteria are described in the Preamble to the IARC Monographs, which is available on the IARC Monographs website:
https://monographs.iarc.fr/wp-content/uploads/2019/07/Preamble-2019.pdf.
Experts critically review four types of data:
. the situations in which people are exposed to the agent;
. epidemiological studies of cancer in humans exposed to the agent (scientific evidence of carcinogenicity in humans);
. experimental studies of cancer in laboratory animals treated with the agent (scientific evidence of carcinogenicity in experimental animals); and
. studies on how cancer develops in response to the agent (scientific evidence on mechanisms of carcinogenesis).
7. What are IARC’s recommendations based on these results?
IARC is a research organization that evaluates the evidence on the causes of cancer but does not make health recommendations. However, the evaluations made by the IARC Monographs are often used as a basis for national and international policies, guidelines, and recommendations to minimize cancer risks.
Infographic
Visit the webpageVolume 127 webpage