More
26 March 2021
IARC Monographs Meeting 129: Gentian Violet, Leucogentian Violet, Malachite Green, Leucomalachite Green, and CI Direct Blue 218
QUESTIONS AND ANSWERS (Q&A)
 IARC Monographs Meeting 129
Home
IARC Monographs Meeting 129
HomeThe meeting for IARC Monographs Volume 129: Gentian Violet, Leucogentian Violet, Malachite Green, Leucomalachite Green, and CI Direct Blue 218, convened by the International Agency for Research on Cancer (IARC) in Lyon, France, and held remotely due to the coronavirus disease (COVID-19) pandemic, took place on 22 February to 5 March 2021.
The Working Group of international experts, including 11 scientists from 8 countries, evaluated the carcinogenicity of five agents: gentian violet, leucogentian violet, malachite green, leucomalachite green, and CI Direct Blue 218.
More information about the meeting is available on the IARC Monographs website: https://monographs.iarc.who.int/iarc-monographs-volume-129/.
The outcome of the assessment has been published in a summary article in The Lancet Oncology[1] and will be described in detail in Volume 129 of the IARC Monographs, to be published in 2022.
[1]IARC Monographs Volume 129 Working Group (2021). Carcinogenicity of gentian violet, leucogentian violet, malachite green, leucomalachite green, and CI Direct Blue 218. Lancet Oncol, Published online 25 March 2021; https://doi.org/10.1016/S1470-2045(21)00178-9
What are the results of the evaluation?
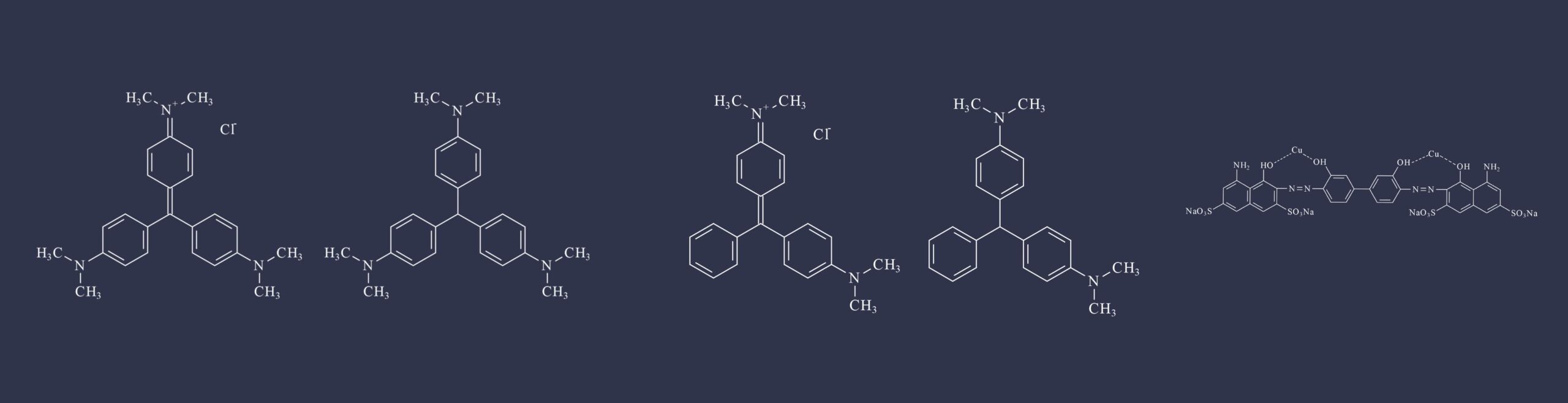
Gentian violet (CAS No. 548-62-9) was classified as Group 2B (possibly carcinogenic to humans) on the basis of sufficient evidence of carcinogenicity in experimental animals. There was limited mechanistic evidence and inadequate evidence regarding carcinogenicity in humans.
Leucogentian violet (CAS No. 603-48-5), a metabolite of gentian violet, was classified as Group 3 (not classifiable as to its carcinogenicity to humans) because the evidence regarding carcinogenicity in humans, the evidence regarding carcinogenicity in experimental animals, and the mechanistic evidence were inadequate; no studies were available.
Malachite green (CAS No. 569-64-2, 14426-28-9, 2437-29-8) was classified as Group 3 (not classifiable as to its carcinogenicity to humans) because the evidence of carcinogenicity in experimental animals and the mechanistic evidence were limited and the evidence regarding carcinogenicity in humans was inadequate.
Leucomalachite green (CAS No. 129-73-7), a metabolite of malachite green, was classified as Group 2B (possibly carcinogenic to humans) on the basis of sufficient evidence of carcinogenicity in experimental animals. The mechanistic evidence was limited, and the evidence regarding carcinogenicity in humans was inadequate.
CI Direct Blue 218 (CAS No. 28407-37-6) was classified as Group 2B (possibly carcinogenic to humans) on the basis of sufficient evidence of carcinogenicity in experimental animals. The evidence regarding carcinogenicity in humans and the mechanistic evidence were inadequate.
Who is exposed to these agents, and how?
Gentian violet is used as a textile dye or ink, as a non-commercial hair dye, and as a constituent of other cosmetics. It is also used to stain biological specimens. In medicine, gentian violet can be used to treat a variety of bacterial and fungal infections in humans and animals, including fungi and intestinal parasites in fish and poultry, and bacterial and fungal infections of the skin and eye in livestock (topical treatment). In many countries, veterinary or cosmetic applications are restricted, and there is zero tolerance for residues of gentian violet and malachite green and their metabolites, leucogentian violet and leucomalachite green, in food for human consumption.
The available data on human exposure were sparse.
Leucogentian violet is used in analytical chemistry, as a chromogenic reagent for chemical assays, and as a radiochromic indicator (in dosimeters, to measure radiation exposure). It can also be used in forensics (to enhance blood impression evidence from fingerprints and footwear). Dietary exposure to leucogentian violet may occur through fish consumption if gentian violet has been used in aquaculture.
No data were available on human exposure.
Malachite green is used as a dye for materials such as cotton, silk, leather, wool, and paper and as a semi-permanent cosmetic dye for hair and in body oils. It is also used as a biological stain and clinical reagent (in biological assays) and as an aquarium disinfectant. It can be used as an antifungal, antiparasitic, and antibacterial agent, particularly in the aquaculture industry.
The available data on human exposure were sparse.
Leucomalachite green is used as a reagent in forensic science, in analytical chemistry, as a chromogenic reagent for chemical assays, and as a radiochromic indicator (in dosimeters, to measure radiation exposure). Dietary exposure to leucomalachite green may occur through fish consumption if malachite green has been used in aquaculture.
The available data on human exposure were sparse.
CI Direct Blue 218 is used as a dye for cellulose, acetate, paper, fabrics, textiles, and other materials.
No data were available on human exposure.
Have these agents been evaluated previously by the IARC Monographs Programme?
This is the first time that these five agents have been evaluated by the IARC Monographs Programme. However, the Joint Expert Committee on Food Additives (JECFA) of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) conducted risk assessments for gentian violet (in 2013) and for malachite green (in 2009). No Acceptable Daily Intake (ADI) could be established by JECFA for gentian violet and malachite green, because of concerns about the carcinogenicity of these compounds.
How are the evaluations made by IARC and JECFA complementary?
Both JECFA and the IARC Monographs have recognized the carcinogenic potential of gentian violet, malachite green, and their leucometabolites.
The IARC Monographs Programme and the JECFA Programme have different yet complementary scopes. The IARC Monographs Programme identifies environmental factors that are carcinogenic hazards to humans. JECFA is an international scientific expert committee administered jointly by FAO and WHO. JECFA evaluates the safety of food additives, contaminants, naturally occurring toxicants, and residues of veterinary drugs in food. JECFA performs risk assessments and provides essential guidance to FAO, WHO, and the member countries of both organizations, as well as to the Codex Alimentarius Commission.
In 2019, an independent advisory group convened by the IARC Monographs Programme noted the risk assessments by JECFA and recommended that the IARC Monographs Programme evaluate gentian violet, malachite green, and leucomalachite green with high priority. The attendant classification and listing by the IARC Monographs would extend to all routes and types of exposure. The IARC Monographs evaluation therefore built on the JECFA evaluation, while also considering the many other important exposures and use scenarios (e.g. as human medicines and in a range of other settings, including occupational, laboratory, and personal care products, such as hair dyes). In addition, the IARC Monographs considered any new data that had become available since the JECFA evaluations.
The evaluations by IARC and JECFA are complementary. The IARC Monographs Programme classified gentian violet in Group 2B (possibly carcinogenic to humans), and the JECFA risk assessment did not establish an Acceptable Daily Intake (ADI) for this compound, because of concerns about its carcinogenicity. The IARC Monographs Programme classified leucomalachite green (produced in the flesh of fish treated with malachite green) in Group 2B (possibly carcinogenic to humans). Thus, the IARC Monographs classification supports the determination by JECFA that there should be no ADI for malachite green. Both the IARC Monographs hazard evaluation and the JECFA risk assessment considered the same toxicological studies conducted for leucomalachite green.
The JECFA report for gentian violet is available at https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/6189. The JECFA report for malachite green is available at https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/5872.
How was the evidence reviewed in the IARC Monographs evaluation?
During an evaluation by the IARC Monographs Working Group, experts critically review the scientific evidence according to strict criteria, which focus on determining the strength of the available evidence that the agent causes cancer. These criteria are described in the Preamble to the IARC Monographs, which is available here: https://monographs.iarc.fr/wp-content/uploads/2019/07/Preamble-2019.pdf.
Experts critically review four types of data:
- the situations in which people are exposed to the agent;
- epidemiological studies of cancer in humans exposed to the agent (scientific evidence of carcinogenicity in humans);
- experimental studies of cancer in laboratory animals treated with the agent (scientific evidence of carcinogenicity in experimental animals); and
- studies of how cancer develops in response to the agent (scientific evidence on carcinogen mechanisms).
What does the IARC Monographs classification mean in terms of risk?
The IARC Monographs classification indicates the strength of the evidence that a substance or agent causes cancer. The IARC Monographs Programme seeks to identify cancer hazards, meaning the potential for the exposure to cause cancer; however, it does not indicate the level of cancer risk associated with exposure at different levels. The cancer risk associated with substances or agents that are assigned the same classification may be very different, depending on factors such as the type and extent of exposure and the size of the effect of the agent at a given exposure level.
What are the four different categories into which agents are classified by the IARC Monographs?
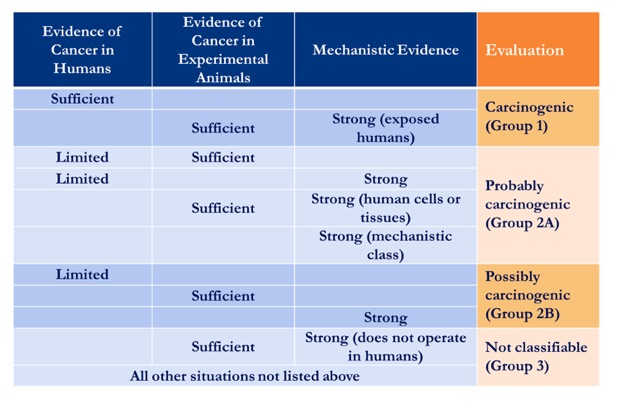
Group 1: The agent is carcinogenic to humans
This category is used when there is sufficient evidence of carcinogenicity in humans. In other words, there is convincing evidence that the agent causes cancer in humans. The evaluation is usually based on the results of epidemiological studies showing development of cancer in exposed humans. Agents can also be classified in Group 1 on the basis of sufficient evidence of carcinogenicity in experimental animals supported by strong evidence in exposed humans that the agent exhibits one or more of the recognized key characteristics of human carcinogens.
Group 2:
This category includes agents with a range of evidence of carcinogenicity in humans and in experimental animals. At one extreme of the range are agents with positive but not conclusive evidence of carcinogenicity in humans. At the other extreme are agents for which evidence in humans is not available but for which there is sufficient evidence of carcinogenicity in experimental animals. There are two subcategories, which indicate different levels of evidence.
Group 2A: The agent is probably carcinogenic to humans
This category is used when there is limited evidence of carcinogenicity in humans and either sufficient evidence of carcinogenicity in experimental animals or strong mechanistic evidence, showing that the agent exhibits key characteristics of human carcinogens. Limited evidence of carcinogenicity means that a positive association has been observed between exposure to the agent and cancer but that other explanations for the observations (technically termed “chance”, “bias”, or “confounding”) could not be ruled out with reasonable confidence. This category may also be used when there is inadequate evidence regarding carcinogenicity in humans but both sufficient evidence of carcinogenicity in experimental animals and strong mechanistic evidence in human cells or tissues.
Group 2B: The agent is possibly carcinogenic to humans
This category is generally used when only one of the following evaluations has been made by the IARC Monographs Working Group:
- limited evidence of carcinogenicity in humans
- sufficient evidence of carcinogenicity in experimental animals
- strong mechanistic evidence, showing that the agent exhibits key characteristics of human carcinogens.
Group 3: The agent is not classifiable as to its carcinogenicity to humans
This category is used most commonly when the evidence of carcinogenicity in humans is inadequate, the evidence of carcinogenicity in experimental animals is limited (or inadequate), and the mechanistic evidence is limited (or inadequate). Limited evidence of carcinogenicity in experimental animals means that the available information suggests a carcinogenic effect but is not conclusive.
The different strength-of-evidence evaluation groups are shown in the following table.
What are IARC’s recommendations based on the results of this meeting?
IARC is a research organization that evaluates the evidence on the causes of cancer but does not make health recommendations. However, the evaluations made by the IARC Monographs are often used as a basis for national and international policies, guidelines, and recommendations to minimize cancer risks.
You can find more information on the IARC Monographs evaluation process here: https://monographs.iarc.who.int/wp-content/uploads/2018/07/QA_ENG.pdf